氡
86
Rn
族
18
週期
6
區
p
個質子
電子
個中子
86
86
136
一般屬性
原子序數
86
標準原子質量
[222]
品質編號
222
類別
惰性氣體
顏色
無色
放射性
是
The name was derived from radium; called niton at first, from the Latin word nitens meaning shining
晶體結構
無法計量
歷史
Radon was discovered in 1900 by Friedrich Ernst Dorn in Halle, Germany.
He reported some experiments in which he noticed that radium compounds emanate a radioactive gas.
In 1910, Sir William Ramsay and Robert Whytlaw-Gray isolated radon, determined its density, and determined that it was the heaviest known gas.
He reported some experiments in which he noticed that radium compounds emanate a radioactive gas.
In 1910, Sir William Ramsay and Robert Whytlaw-Gray isolated radon, determined its density, and determined that it was the heaviest known gas.
每個外殼的電子
2, 8, 18, 32, 18, 8
電子排布
[Xe] 4f14 5d10 6s2 6p6
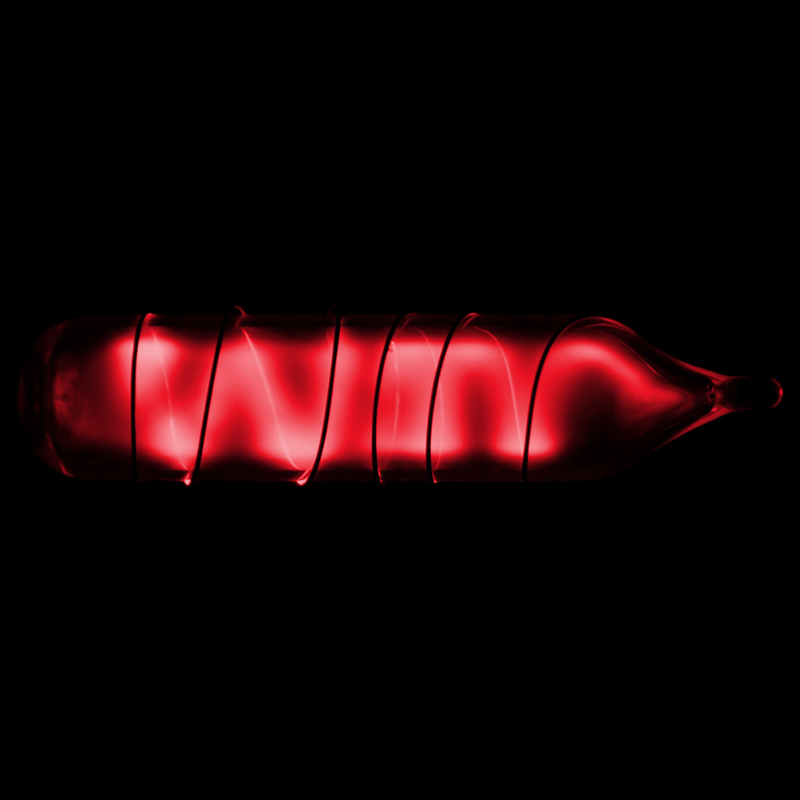
Upon condensation, radon glows because of the intense radiation it produces
物理性質
物態
氣體
密度
0.00973 g/cm3
熔點
202 K | -71.15 °C | -96.07 °F
沸點
211.3 K | -61.85 °C | -79.33 °F
熔化熱
3 kJ/mol
汽化熱
17 kJ/mol
比熱容
0.094 J/g·K
地殼豐度
無法計量
宇宙豐度
無法計量
CAS 編號
10043-92-2
Pubchem CID 編號
24857
原子性質
原子半徑
120 pm
共價半徑
150 pm
電負性
-
電離電位
10.7485 eV
原子體積
50.5 cm3/mol
熱導率
0.0000364 W/cm·K
氧化態
2, 4, 6
應用
Radon is used in hydrologic research that studies the interaction between ground water and streams.
Radon has been produced commercially for use in radiation therapy.
Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers.
Radon has been produced commercially for use in radiation therapy.
Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers.
Radon is highly radioactive and a carcinogen
同位素
穩定同位素
-不穩定同位素
195Rn, 196Rn, 197Rn, 198Rn, 199Rn, 200Rn, 201Rn, 202Rn, 203Rn, 204Rn, 205Rn, 206Rn, 207Rn, 208Rn, 209Rn, 210Rn, 211Rn, 212Rn, 213Rn, 214Rn, 215Rn, 216Rn, 217Rn, 218Rn, 219Rn, 220Rn, 221Rn, 222Rn, 223Rn, 224Rn, 225Rn, 226Rn, 227Rn, 228Rn