鐳
88
Ra
族
2
週期
7
區
s
個質子
電子
個中子
88
88
138
一般屬性
原子序數
88
標準原子質量
[226]
品質編號
226
類別
鹼土金屬
顏色
銀色
放射性
是
From the Latin word radius meaning ray
晶體結構
體心立方
歷史
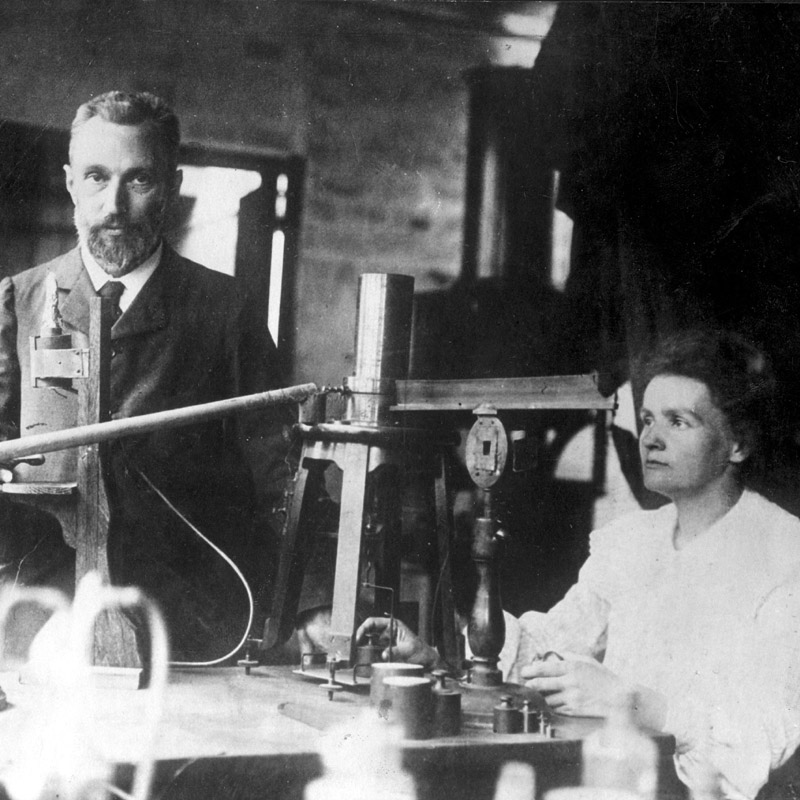
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
每個外殼的電子
2, 8, 18, 32, 18, 8, 2
電子排布
[Rn] 7s2
Radium imparts a carmine red color to a flame
物理性質
物態
固體
密度
5.5 g/cm3
熔點
973.15 K | 700 °C | 1292 °F
沸點
2010.15 K | 1737 °C | 3158.6 °F
熔化熱
8 kJ/mol
汽化熱
125 kJ/mol
比熱容
- J/g·K
地殼豐度
9.9×10-12%
宇宙豐度
無法計量
CAS 編號
7440-14-4
Pubchem CID 編號
6328144
原子性質
原子半徑
-
共價半徑
221 pm
電負性
0.9 (鲍林标度)
電離電位
5.2784 eV
原子體積
45.20 cm3/mol
熱導率
0.186 W/cm·K
氧化態
2
應用
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
同位素
穩定同位素
-不穩定同位素
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra