鉳
97
Bk
族
無法計量
週期
7
區
f
個質子
電子
個中子
97
97
150
一般屬性
原子序數
97
標準原子質量
[247]
品質編號
247
類別
錒系元素
顏色
無法計量
放射性
是
Named after Berkeley, California, the city of its discovery
晶體結構
簡單六角形
歷史
Berkelium was discovered by Glenn T. Seaborg, Albert Ghiorso and Stanley G. Thompson in 1949 at the University of California, Berkeley.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
每個外殼的電子
2, 8, 18, 32, 27, 8, 2
電子排布
[Rn] 5f9 7s2
Just over one gram of berkelium has been produced in the United States since 1967
物理性質
物態
固體
密度
14.78 g/cm3
熔點
1259.15 K | 986 °C | 1806.8 °F
沸點
3173.15 K | 2900 °C | 5252 °F
熔化熱
無法計量 kJ/mol
汽化熱
無法計量 kJ/mol
比熱容
- J/g·K
地殼豐度
無法計量
宇宙豐度
無法計量
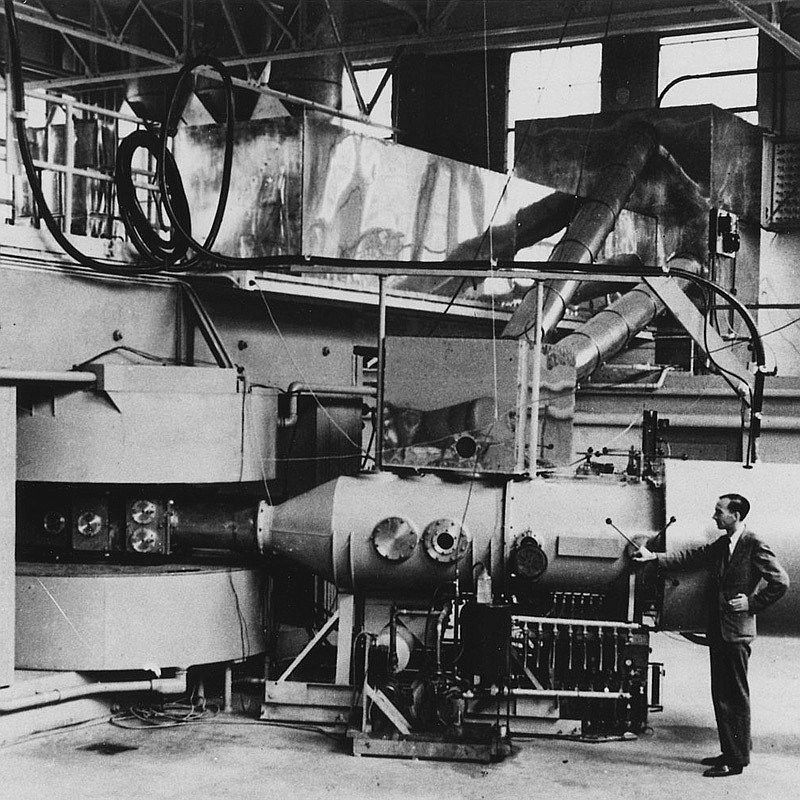
圖片來源: Wikimedia Commons (Department of Energy - Office of Public Affairs)
The 60-inch cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley
CAS 編號
7440-40-6
Pubchem CID 編號
23971
原子性質
原子半徑
170 pm
共價半徑
-
電負性
1.3 (鲍林标度)
電離電位
6.1979 eV
原子體積
16.7 cm3/mol
熱導率
0.1 W/cm·K
氧化態
3, 4
應用
Berkelium is mainly used for scientific research purposes.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Berkelium is harmful due to its radioactivity
同位素
穩定同位素
-不穩定同位素
233Bk, 235Bk, 236Bk, 237Bk, 238Bk, 239Bk, 240Bk, 241Bk, 242Bk, 243Bk, 244Bk, 245Bk, 246Bk, 247Bk, 248Bk, 249Bk, 250Bk, 251Bk, 252Bk, 253Bk, 254Bk