釙
84
Po
族
16
週期
6
區
p
個質子
電子
個中子
84
84
126
一般屬性
原子序數
84
標準原子質量
[210]
品質編號
210
類別
類金屬
顏色
銀色
放射性
是
Named after Poland, native country of Madam Curie
晶體結構
簡單立方
歷史
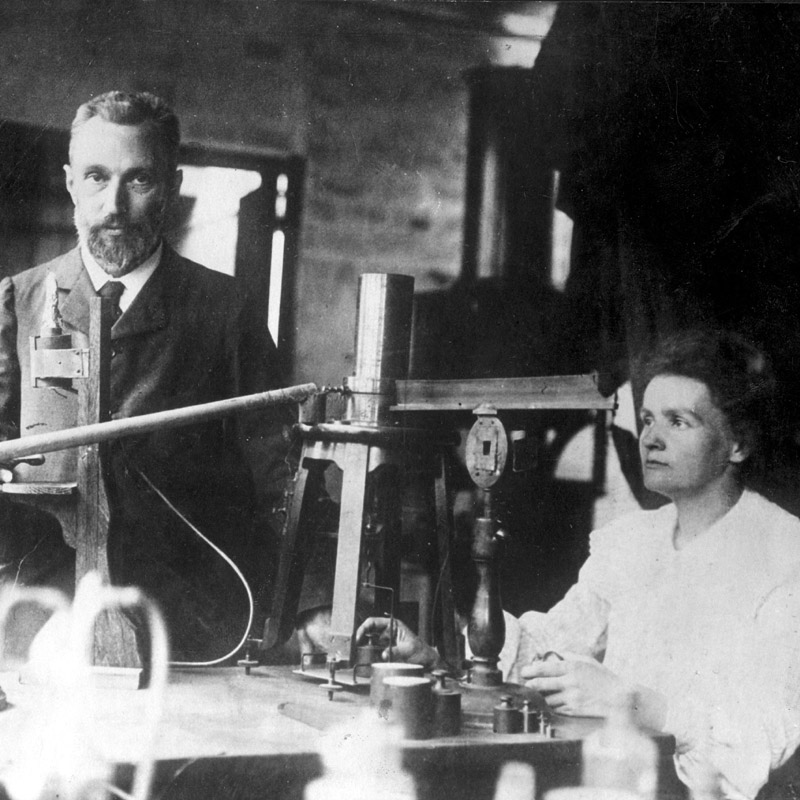
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
每個外殼的電子
2, 8, 18, 32, 18, 6
電子排布
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
物理性質
物態
固體
密度
9.196 g/cm3
熔點
527.15 K | 254 °C | 489.2 °F
沸點
1235.15 K | 962 °C | 1763.6 °F
熔化熱
13 kJ/mol
汽化熱
100 kJ/mol
比熱容
- J/g·K
地殼豐度
無法計量
宇宙豐度
無法計量
CAS 編號
7440-08-6
Pubchem CID 編號
無法計量
原子性質
原子半徑
168 pm
共價半徑
140 pm
電負性
2.00 (鲍林标度)
電離電位
8.417 eV
原子體積
22.23 cm3/mol
熱導率
0.2 W/cm·K
氧化態
-2, 2, 4, 6
應用
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
同位素
穩定同位素
-不穩定同位素
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po